Product Categories
- Related Products (0)
- Main products (5)
- Minoxidil (1)
- Finasteride (1)
- Dutasteride (1)
- RU58841 (1)
- Way316606 (1)
- New Research (4)
- CB-03-01 (1)
- KX-826 (1)
- SM04554 (1)
- Pyrrolidinyl Diaminopyrimidine (1)
KX-826, a new drug in development for the treatment of androgenic alopecia undergoing clinical trials in both China and the United States, treats hair loss by antagonizing the action of androgen receptors in a different mode of action than any of the existing FDA-approved drugs for AGA alopecia. Let’s look forward to this new treatment for hair loss.
KX-826, a non-steroidal androgen receptor antagonist, is a drug under development by Chinese research and development company Kaixin Pharmaceuticals. The company is developing it for the treatment of androgenic alopecia and acne, of which acne is in Phase II clinical trials and androgenic alopecia is in a second phase III trial in China. AGA Phase II clinical trials in the United States have been completed and Phase III clinical trials are being prepared.
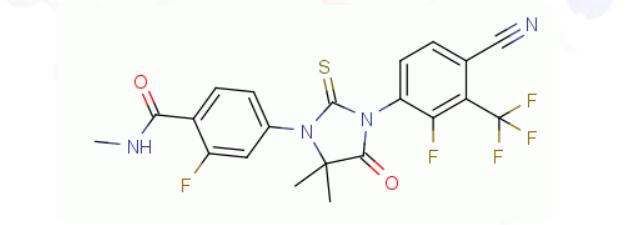
The action principle of KX-826 in the treatment of male pattern alopecia is related to androgens.
Male pattern alopecia (AGA) is also known as male androgenic alopecia, indicating that male baldness is related to the androgen produced by male secretion. Androgens in the male body have two kinds of testosterone and dihydrotestosterone (DHT), DHT and androgen receptor binding affinity is 5 times that of testosterone, therefore, compared with testosterone, DHT is more likely to cause hair loss, so sometimes AGA is also called DHT hair loss.
Drugs already approved by the FDA to treat androgenic alopecia are Minoxidil and finasteride, in which finasteride treats hair loss by inhibiting the action of 5AR and reducing circulating levels of DHT. Finasteride, which is used orally, reduces DHT levels throughout the body and has greater side effects in men, including effects on libido and erectile function. KX-826 is a topical antiandrogen that treats scalp hair loss by blocking the action of androgens (including DHT and testosterone) in the scalp area, more targeted and with fewer side effects. In the trials that have been conducted, KX-826 does not affect men’s libido. At the same time, as a topical drug, KX-826 can also treat female androgenic alopecia.
KX-826 has undergone a number of clinical trials and has achieved positive therapeutic results.
First, let’s look at the clinical trial timeline of KX-826, as follows:
| clinical trial | Participant | status |
| Phase II clinical trial | Chinese male AGA | It was completed and reached its primary endpoint in November 2022. |
| Phase II clinical trial | Chinese female AGA | It was completed and reached its primary endpoint in November 2022. |
| Phase II clinical trial | American male AGA | Completed and reached the primary endpoint in May 2023. |
| Phase III clinical trial | Chinese male AGA | The first subject was enrolled for treatment in January 2022 and all subjects were enrolled for treatment in March 2023. In November 2023, it was announced that top-line data had been read from the Phase III clinical trial. |
| Phase III clinical trial | Chinese male and female AGA | Recruitment underway. In July 2023, the first patient was enrolled. |
In the Phase II clinical trial of AGA in men in China, a total of 120 Chinese participants had moderate or more symptoms of AGA alopecia. Patients were treated with 0.25% KX-826 solution and 0.5% KX-826 solution in two different concentrations, one group received 0.25% KX-826 solution twice daily, and the other group received 0.5% KX-826 solution once daily. One group received 0.5% KX-826 solution twice daily.
After 24 weeks, men in the twice-daily 0.5% KX-826 solution group had the best hair improvement, with an increase of 22.73 hairs per square centimeter from baseline and 15.34 hairs per square centimeter compared to the placebo group.
The push dose of AGA Phase III clinical trial in Chinese men was determined to be 0.5% KX-826 solution twice daily (i.e., 5mg twice daily for a total of 10mg).
The Phase II clinical trial of AGA in women in China included 160 female participants, all of whom had moderate to severe symptoms of female pattern alopecia and were treated for 24 weeks. Patients were treated with 0.25% and 0.5% of KX-826 solution at different concentrations, and 119 female patients were randomly assigned to one of four treatment groups: The first group received 0.25% KX-826 solution once daily, the second group received 0.25% KX-826 solution twice daily, and the third group received 0.5% KX-826 solution once daily. The fourth group received 0.5% KX-826 solution twice daily.
After 24 weeks, the group treated with 0.5% KX-826 solution once a day had 11.39 more hairs per square centimeter than the base group. In addition, efficacy was shown as early as the end of week 12. In the test, the overall safety is good. Most of the adverse events that occurred during treatment were mild and similar to placebo.
The recommended dose for the Phase III clinical trial in Chinese women was determined to be 0.5% KX-826 solution once daily (i.e., 5mg once daily).
In the Phase II clinical trial of AGA in men conducted in the United States, there were 123 male AGA patients, of which 93 patients were randomly assigned to one of three groups. Receiving 0.25% KX-826 solution once daily, 0.5% KX-826 solution once daily and 0.5% KX-826 solution twice daily, another 30 patients were randomly assigned to receive different doses of placebo.
After 24 weeks, the 0.5% KX-826 solution group achieved significant hair improvement, with an increase of 10 hairs per square centimeter compared to baseline.
As in the Chinese Phase II trial, 0.5% KX-826 solution twice daily was confirmed as the optimal dose, and the recommended dose for the AGA Phase III trial in men in the United States was determined to be 0.5% KX-826 solution twice daily (i.e., 5mg twice daily for a total of 10mg).
The randomized, double-blind, placebo-controlled, multi-regional Phase III trial in China involved 416 participants and lasted 24 weeks. The Phase III trial will evaluate the efficacy and safety of KX-826 treatment.
The dose used in the Phase III trial was 0.5% KX-826 solution twice daily. Kintor announced in November 2023 that its Phase III clinical trial has read top-line data. The results show that the overall safety is excellent. The KX-826 group effectively promoted hair growth, which was statistically significant. Treatment with KX-826 showed a therapeutic effect compared to the placebo group, but did not achieve statistical significance.
In July 2023, Kintor is also conducting a 52-week, 16-center Phase III clinical trial of KX-826, which includes both male and female hair loss patients and is said to be still enrolling participants.
Phase II clinical trials in Chinese men, Chinese women, and U.S. men all demonstrated the effectiveness of KX-826 in the treatment of male pattern baldness (AGA) and determined the dosage of KX-826.
Among them, in the men’s AGA II trial conducted in China and the United States, the number of Chinese men’s hair increased to 22.73, and the number of American men’s hair increased to 10, there is a certain difference, I would like to see the data of the last phase III experiment to see if the data would change.
Data from the Phase III trial in China is not yet available, and trials are still underway in both male and female AGA patients, looking forward to seeing final trial data.
KX-826 has enough experimental data to validate its dosage, and although clinical trials have not been fully completed, its actual use dose is established.
The optimal dose for men is 5mg twice daily (5% KX-826 solution).
The dose for women is 5mg once daily (5% KX-826 solution).
This is the best dose to be tested, and the actual dose will vary from person to person, or you can start with a small dose, such as 2.5mg per day.
The side effects of KX-826 in the experiment were small, and the most common were itching and inflammation at the contact site.
Seeing KX-826 for both androgenic alopecia and acne, did you think of a similar drug? Yes, it’s CB-03-01, also for acne and AGA hair loss.
KX-826 and CB-03-01, which share the same adaptive symptoms, are both used to treat acne and androgenic alopecia, and both are currently in clinical trials. In the treatment of AGA, their mode of action is actually the same, both target androgen receptors, and treat androgenic alopecia by blocking the action of androgens.
KX-826 was developed by China Kintor Pharmaceutical.
CB-03-01 was developed by Cassiopeia, an Italian pharmaceutical company.
Phase II clinical trials for CB-03-01 have been completed and Phase III clinical trials are scheduled to begin in June 2023.
The Phase II clinical trial of KX-826 has been completed in China and the United States, with the first Phase III clinical trial in Chinese male AGA completed in November 2023 and the second Phase III clinical trial in Chinese male and female AGA beginning in July 2023.
The dose of CB-03-01 solution is 1.5ml 5% BID, that is, 75mg in a single dose and 150mg in a day.
The dose of KX-826 solution is 1ml 0.5% BID, that is, 5mg in a single dose and 10mg in a day.
In the Phase II clinical trial of cb-03-01, 7.5% applied twice daily was the most effective, resulting in an increase of 20.79 hairs per square centimeter after 6 months and 14.3 hairs per square centimeter after 12 months. After 6 months of 5 percent daily use, the hair had increased by 12.21 hairs and after 12 months by 13.8 hairs.
In the Chinese Phase II clinical trial of KX-826, two 0.5% treatments per day resulted in an increase of 22.73 hairs per square centimeter after 24 weeks. In the US Phase II clinical trial, after 24 weeks of 0.5% treatment twice a day, the hair increased by 10 hairs.
KX-826 is ahead of CB-03-01 in the development of KX-826 and CB-03-01, although KX-826 was not significant compared to placebo in the most recently published AGA Phase III trial in men, KX-826 is still being conducted in both men and women. With longer validation trials, Kintor still have a lot of confidence in the KX-826.
The KX-826, which is undergoing several clinical trials in both China and the United States, is still ahead of cb-03-01 in the experimental schedule, and perhaps, if it is eventually approved, the KX-826 will be slightly earlier than cb-03-01.
From the dose point of view, KX-826 is 10mg per day, and cb-03-01 is 150mg per day. KX-826 can achieve the effect of cb-03-01 in a small dose, which may save money.
Among several hair loss drugs under study, KX-826 treats hair loss by antagonizing androgen action, which has a different mode of action from drugs already approved by the FDA to treat hair loss, and will be safer for topical use with fewer side effects, which is a new direction for the treatment of AGA. Finasteride is only approved for male hair loss, and KX-826, if approved, would add another treatment for female hair loss.
Male baldness, also known as male androgenic alopecia, indicates that the main cause of baldness is androgens. However, Minoxidil does not reduce the level of androgens and is also a good treatment for hair loss. There is also Way-316606 to treat hair loss by enhancing the action of WNT. This suggests that the causes of hair loss are complex, from genetics to lifestyle habits that determine the density of our hair. In a double-blind trial to treat hair loss, the placebo group did not know whether they were taking KX-826 or a placebo, but when they maintained good sleep and lifestyle habits, they lost less hair and even increase hair than at baseline. Therefore, hair loss is caused by a variety of factors. KX-826 is a new mode of action that significantly improves hair condition when used alone, and when combined with Finasteride or Minoxidil, or other hair loss medications, improves hair loss ina variety of ways.