Product Categories
- Related Products (0)
- Main products (5)
- Minoxidil (1)
- Finasteride (1)
- Dutasteride (1)
- RU58841 (1)
- Way316606 (1)
- New Research (4)
- CB-03-01 (1)
- KX-826 (1)
- SM04554 (1)
- Pyrrolidinyl Diaminopyrimidine (1)
CB-03-01, also known as Clascoterone, is being developed as a solution for the treatment of male androgenic alopecia and is currently the most promising for FDA approval anti-hair loss hair growth drug.
CB-03-01 is the development code name for clascoterone, also known as Breezula.
Clascoterone has already been approved by the FDA for the treatment of acne in 2020. It’s called Winlevi.
CB-03-01 is an anti-androgen drug used to treat acne and to treat hair loss both by blocking or reducing the action of androgens, just in different concentrations.
When it is used to treat acne, it is applied in the form of a 1% cream or cream. When it was developed to treat hair loss, it was in solution form and in higher concentrations.
CB-03-01 is an antiandrogen that prevents androgens from binding to androgen receptors. The androgens in the male body are mainly testosterone and dihydrotestosterone (DHT), and these androgens bind to the androgen receptors in the hair follicle and play a role in the hair follicle, thus affecting the health of the hair follicle and hair growth.
Why use CB-03-01 for Acne? This is because some acne is caused by androgens. During male adolescence, it is more likely to get acne because androgen levels are high during this period.
The male sex hormone testosterone is converted into the more androgenic DHT by the action of 5A reductase, which is found in large quantities in the skin and hair follicles, so testosterone is converted into large amounts of DHT in the skin and hair follicles. Androgens are known to be the main cause of hair loss and acne, so a lot of DHT and testosterone accumulate in the skin and hair follicles, produce a lot of oil, cause inflammation, clog pores, etc., will cause acne.
CB-03-01 treats acne by reducing the buildup of androgens outside the skin and hair follicles. CB-03-01 is an FDA-approved drug for the treatment of acne, and its safety and efficacy have been tested in numerous trials.
It is known that androgenic alopecia is mainly associated with DHT. The only oral anti-hair loss medication approved by the FDA is Finasteride, which prevents hair loss by reducing the production of DHT.
Both CB-03-01 and Finasteride are DHT-related, but the mode of action is completely different.
Finasteride reduces circulating DHT levels by inhibiting the action of 5A reductase so that testosterone cannot be converted into androgens.
CB-03-01 antagonizes the action of androgens (DHT AND testosterone), so that it cannot play its corresponding role in the corresponding site.
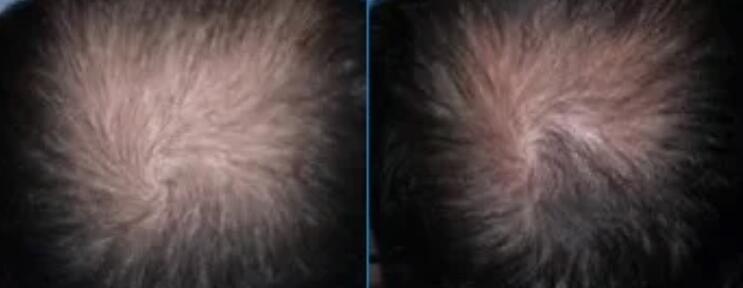
CB-03-01 is a drug under development for the treatment of androgenic alopecia.
Phase II clinical trial of CB-03-01 was completed in 2019, and the Phase II trial validated the dosage of CB-03-01. The Phase III trial was previously expected to start in 2022, possibly due to COVID-19, but was officially announced to start in June 2023.
The Phase III trial of CB-03-01 is expected to last one year, involve 726 participants and is expected to be completed in 2025. The main objective was to verify the efficacy, safety and tolerability of 5% CB-03-01 solution in the treatment of male pattern baldness.
If CB-03-01 completes Phase III clinical trials and receives FDA approval, it would be the third FDA-approved treatment for androgenic alopecia after Minoxidil and Finasteride. It will also be the first topical AGA drug to be used for the purpose of antagonizing androgen effects.
At present, we can see the Phase II trial data of CB-03-01. The Phase II trial validated the dose range of CB-03-01 solution and compared therapeutic efficacy and safety. There are four verified CB-03-01 solutions, which are 2.5% twice a day, 5% twice a day, 7.5% twice a day and 7.5% once a day.
Six months after the trial began, an interim Phase II analysis report for CB-03-01 was published, with the following data:
| Six Month Interim Analysis efficacy Results (N=375) | Twice daily CB-03-01 solution 2.5% | Twice daily CB-03-01 solution 5% | Twice daily CB-03-01 solution 7.5% | Once a day CB-03-01 solution 7.5% | Vehicle |
| Mean changes from baseline(target area hair count) | 13.01 | 12.21 | 20.79 | 11.51 | -0.11 |
| Favorable hair growth assessment (+1,+2,+3) | 56% | 58% | 62% | 61% | 49% |
The trial showed that using CB-03-01 to treat male pattern hair loss, four different concentrations of CB-03-01 solution were able to help hair growth compared to the placebo group, and the placebo group had more hair loss.
Of these four different concentrations, 7.5% CB-03-01 solution twice daily worked best.
| twelve Month Interim Analysis efficacy Results (N=344) | Twice daily CB-03-01 solution 2.5% | Twice daily CB-03-01 solution 5% | Twice daily CB-03-01 solution 7.5% | Once a day CB-03-01 solution 7.5% | Vehicle |
| Mean changes from baseline(target area hair count) | 10.2 | 13.8 | 14.3 | 12.7 | 0 |
| Favorable hair growth assessment (+1,+2,+3) | 60.8% | 60.6% | 61.8% | 56.1% | 50% |
In the above test data, the effect of 7.5% CB-03-01 liquid is still better twice a day.
However, in the previous Phase III trial, we can see that Cassiopeia selected 5% CB-03-01 liquid. Why not use 7.5%?
The answer is also in the trial data. Using 7.5% CB-03-01 liquid twice a day and once a day, the actual hair growth effect decreased slightly at 6 months and 12 months, while the 5% CB-03-01 liquid at 6-12 months showed the actual effect was still increasing.
Therefore, Cassiopeia finally chose the 5% CB-03-01 liquid.
In fact, in the Phase III trial, Cassiopeia chose 1.5ml 5% CB-03-01 solution twice daily, and did not lower the daily dose, but made up the dose by increasing the application amount.
The Phase III trial of 5% CB-03-01 solution for male pattern baldness is ongoing. There are currently two FDA-approved drugs for androgenic alopecia, the first being topical Minoxidil, approved in 1988, and the second being oral Finasteride 1mg, approved in 1997. It has been almost 30 years and no new treatment for AGA has been approved.
The main cause of AGA is DHT, and Finasteride officially works by reducing the production of DHT. Minoxidil doesn’t interfere with DHT production or reduce circulating levels of DHT, but it’s still effective at aiding hair growth, showing that there are actually multiple causes of hair loss.
CB-03-01 treats hair loss ina different mode of action than either Minoxidil or Finasteride, making it an entirely new treatment. Finasteride is more effective when combined with Minoxidil, then, CB-03-01 is combined with them ina different pattern to prevent scalp hair loss and restore scalp health.
CB-03-01, which has a different mode of action than the two drugs already approved for male pattern baldness, has entered Phase III clinical trials, where it has fewer side effects, and is already FDA-approved for acne. In summary, it is a promising new drug for AGA treatment.
Cassiopeia, an Italian pharmaceutical company owned by Cosmo Pharmaceuticals, is a relatively small company, but it is primarily focused on dermatology and is developing treatments for acne, genital warts and androgenic alopecia.
As a result, they tend to focus more resources on major projects than other large companies developing multiple drugs at the same time, and there may not be enough investment and attention for smaller projects.
If Cassiopeia focuses on the development of CB-03-01 liquid, we believe we can see the results more quickly.
CB-03-01 as a local topical drug, basically does not enter the human blood circulation system, so it will not interfere with the normal level of hormones in the human body, especially testosterone. In clinical trials, no side effects were found in men’s sexual performance. finasteride and Dutasteride both reduce the level of circulating DHT, which has a greater impact on male sexual desire and sexual function. CB-03-01 liquid can be applied to the location of hair loss with good effect and little side effects.
After topical application, CB-03-01 rapidly hydrolyzes to cortisol. Cortisol is a hormone secreted by the body and is therefore a safe metabolite. Cortisol has anti-inflammatory activity and can relieve scalp inflammation, which is beneficial to the health of the scalp and hair.
The Phase II trial validated CB-03-01 in doses ranging from 2.5% to 7.5%, and the final Phase III trial selected a 5% concentration of CB-03-01 liquid for 1.5ml twice daily use.
The 5% CB-03-01 liquid contains 50mg CB-03-01 powder per 1ml liquid, and the 1.5ml liquid contains 75mg CB-03-01 powder. The dose of CB-03-01 is 75mg twice daily.
If you need to prepare 60ml 5% CB-03-01 liquid, you need 3g CB-03-01 powder.
If you need to make 60ml 2.5% CB-03-01 liquid, you need 1.5g CB-03-01 powder.
Local skin irritation: The most common side effect reported by CB-03-01 was mild to moderate skin irritation at the site of application. This includes symptoms such as redness, itching, burning or dryness. These side effects are usually temporary and gradually subside as the skin adjusts to the drug.
Allergic reactions: Although rare, some people may be allergic to CB-03-01. Symptoms include severe itching, hives, swelling or difficulty breathing. If you notice any signs of an allergic reaction, discontinue use immediately and seek medical attention.
Eye irritation: Care should be taken to avoid contact with eyes when using CB-03-01. If the medication comes into contact with the eyes, it may cause eye irritation, redness, or discomfort.
Dry and peeling: Some users may experience dry and peeling skin at the site of use. This can be managed by using a moisturizer or adjusting the frequency of application as directed by a healthcare professional.
HPA axis inhibition: CB-03-01 is metabolized into cortisol, which may affect its own cortisol secretion, cortisol is regulated by the hypothalamic-pituitary-adrenal axis (HPA axis), and when cortisol levels are elevated, it will inhibit the action of the HPA axis and reduce endogenous cortisol secretion. This is similar to HPTA(hypothalamic-pituitary-testis) secretion with endogenous testosterone.